New results presented this week at the EGU General Assembly have scientists adjusting the assumptions that have longed acted as bookends on the way we understand the evolution of planetary atmospheres. On one side, researchers have identified a previously unrecognised greenhouse effect that could have warmed the early Earth. On the other, the Curiosity rover has uncovered evidence that Mars has lost up to 95% of its original atmosphere, resolving a lost-standing discrepancy between previous estimates and confirming that conditions in the past could have been much more hospitable to life than they are today. Together, these new insights show how humble gases like hydrogen, nitrogen and argon hold the key to some of the great mysteries of the universe.
The early Earth continues to fascinate our imaginations, evoking images of seething volcanoes and fiery meteorites raining down on barren landscapes. It also continues to confound efforts by scientists to understand a seemingly straightforward question: how come the entire planet was not swaddled in ice? In 1972, the famous cosmologist, Carl Sagan and his colleague, George Mullen, posed what came to be known as the Faint Young Sun Paradox: if our sun is like other stars, it should have been about 30% weaker 4 billion years ago than it is today. Such a dimming of our solar furnace should have plunged our planet into global glaciation. But among the few pieces of geologic evidence to survive from the early Earth are unequivocal signs of liquid water. How can this be?
Researchers have proposed numerous mechanisms to warm the Earth, including insulating it with high levels of greenhouse gases or reducing the amount of solar radiation reflected back to space by small, young continents. Both hypotheses have merit, but they have also been plagued by complications. New work by Robin Wordsworth and Raymond Pierrehumbert at the University of Chicago presented at EGU on Tuesday raises yet another possibility: the very different composition of the early atmosphere may have allowed inert gases like H2 and N2 to act like powerful greenhouse gases.
The modern atmosphere lacks hydrogen because these light molecules easily defy Earth’s gravitational pull and escape into space. However, this loss takes time, and Wordsworth believes that the atmosphere could have been rich in hydrogen 3.8 billion years ago, the era of the Faint Young Sun. At this time, the atmosphere also contained two to three times more nitrogen than today — an inference deduced by the abundance of nitrogen now stored in the Earth’s mantle that does not appear to have originated there. Full of H2 and N2, the atmosphere could have experienced some unexpected phenomena.
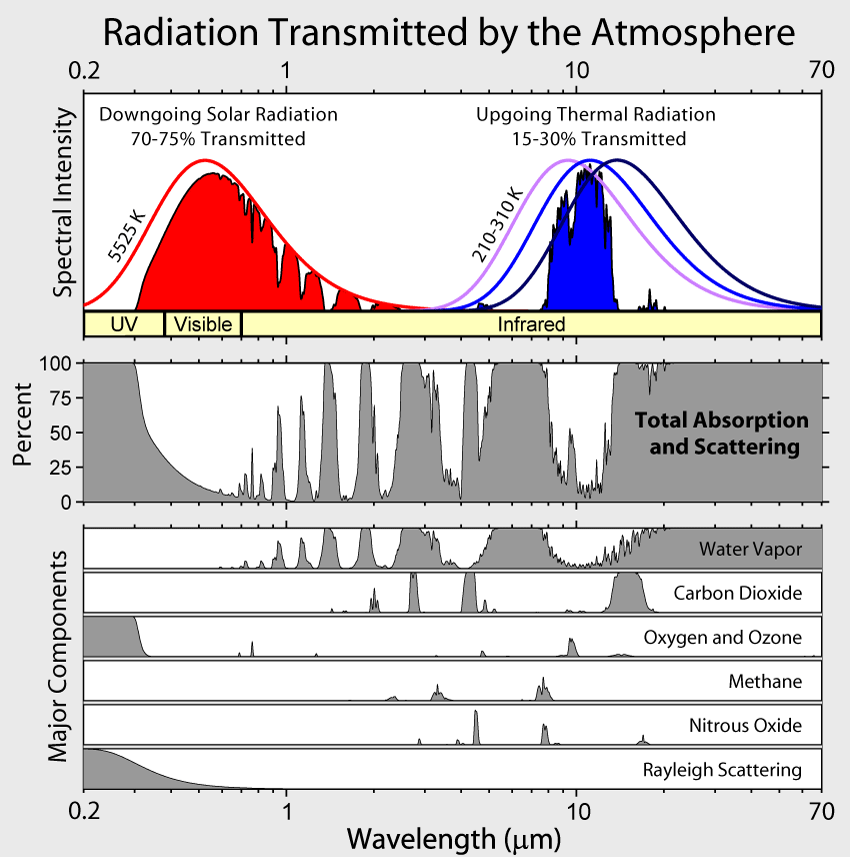
The atmosphere allows most incoming solar radiation to reach the surface, but traps outgoing radiation in the bands of shaded grey. Most of the long wave radiation that cools the Earth escapes through the “water vapor window” centered around 10 um. Source: Wikimedia Commons.
H2 and N2 do not absorb radiation on their own, but at high concentrations they can act like greenhouse gases when they interact. “There are two basic processes,” explains Wordsworth, “first, H-N2 dimers can have additional vibrational and rotational modes, and allow for absorption in new regions of the spectrum. You can also have intense collisions between molecules that can allow previously forbidden transitions to occur.” The end result is that H2-N2 interactions can allow absorption at frequencies inside the so-called water vapor window, a region of the spectrum where outgoing radiation can escape to space and cool the Earth without being trapped by water vapor, the most abundant greenhouse gas of all. For this reason, says Wordsworth, “a little bit of absorption can have a big effect.”
In the conditions likely to have prevailed on the early Earth, their new study suggests the H2-N2 effect could account for 10-15°C of warming. And while this is not enough to explain the Faint Young Sun Paradox by itself, it can help if several mechanisms are allowed to work in tandem. As is often the case in nature, there may be no single “smoking gun”. In fact, Wordsworth thinks it may be time to shift how we think about the Faint Young Sun Paradox altogether. “The Faint Young Sun problem is important as a motivation for studying the early Earth”, he says, “but there is also the broader, more long-term aim of just understanding what the climate was like”.
The habitable zone is defined as the range of distances from a host star that planets could be warm enough to host life. Source: Wikimedia Commons.
With this expansive mindset, Wordsworth has also started extending the H2-N2 mechanism beyond our own planet to understand what conditions might be like on exoplanets and whether or not they could host life. “The habitable zone is defined as a runaway water vapor greenhouse on the inner edge and the maximum possible greenhouse you can have from CO2 alone on the outer edge,” says Wordsworth of the traditional conception of planets where life might thrive. In light of this new greenhouse effect, the definition of the outer edge has grown blurry; planets that once seemed frigid could now be balmy. Now, he says, “You can have a greenhouse that is very efficient at warming very far away from the host star”.
But you don’t have to look all the way to exoplanets to find a place where the H2-N2 effect might have significant implications; Mars presents a great opportunity closer to home. While Wordsworth was unable to comment on the details of this situation, he confirmed that such a mechanism could indeed be important. “Thinking of additional greenhouse effects that we’ve ignored is very important for early Mars because the Faint Young Sun problem is much harder to explain there. There’s a lot of thinking going on at the moment about how we can get to situations where we increase surface temperatures so you get transient periods of liquid water or episodic melting.”
Surish Atreya of the University of Michigan, on the other hand, was eager to share new results about the modern Martian atmosphere at an EGU press conference showcasing updates from the Curiosity rover on Monday. Curiosity carries a suite of instruments onboard collectively known as SAM, for Sample Analysis at Mars. One of these instruments is a mass spectrometer that recently measured the ratio of heavy and light isotopes of argon in the Martian atmosphere (isotopes are molecules which differ in the numbers of neutrons and their atomic weight). Their new results, which achieve unprecedented precision for an argon measurement on Mars, help clear up a long-standing question: how much of its atmosphere has Mars lost over time?
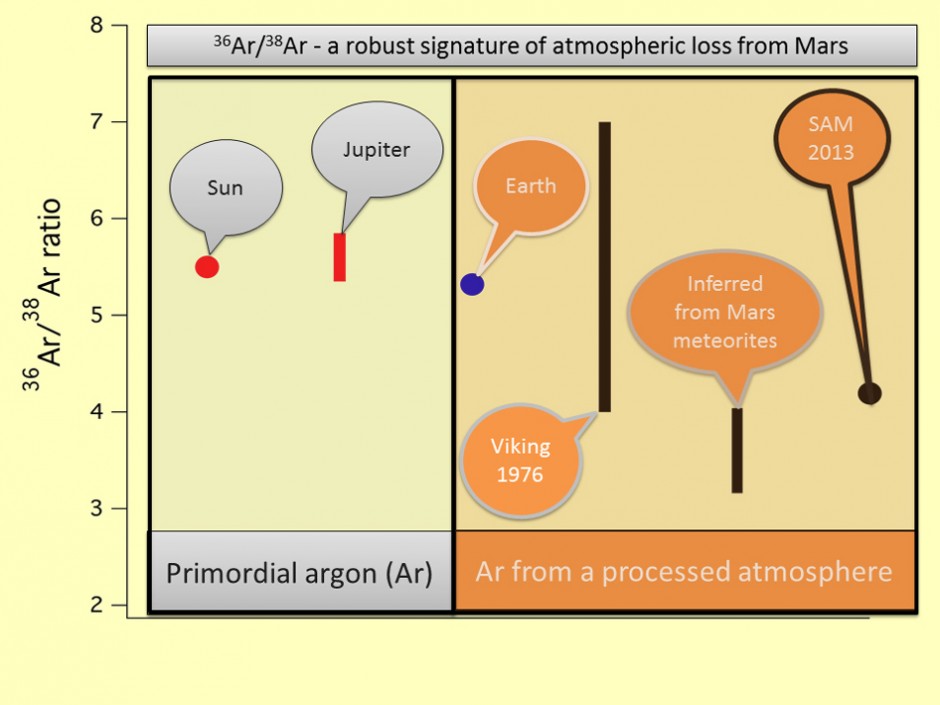
New results from the Curiosity rover show argon ratios that indicate that Mars has lost most of its original atmosphere. Source: NASA/JPL-Caltech
“Argon isotopes are the clearest indication of atmospheric loss”, explained Dr. Atreya. This is because Argon does not participate in any reactions – it is an inert noble gas – so any deviation from the expected ratio must be due to preferentially losing the light isotope to space. Previous measurements from the early Viking rover and from Martian meteorites gave conflicting results, suggesting Mars could have lost all or none of its atmosphere. The new results from SAM show that Mars has probably lost 85-95% of its original atmosphere, a conclusion supported by similarly disproportionate abundances of the heavy isotopes of carbon and oxygen. While these results are not proof of life on Mars, they at least suggest that conditions could have been much friendlier early in the planet’s history when its atmosphere was thicker.
Together, these studies show how simple gases like hydrogen and nitrogen may have shaped the evolution of our planet, and how argon may contain the secrets of another. They reveal how much a planet can change over its history, and how we might need to rethink the way we understand planets far from home. All in all, it seems as though the universe has been, and might be, growing more hospitable by the day.
By Julia Rosen, Freelance science writer and PhD student in Earth, Ocean, and Atmospheric Sciences at Oregon State University


Samuel Illingworth
This is one of the most insightful and interesting blog posts in my field of research (Atmospheric Science) that I have read in a long time. It informs without being patronising, and communicates a number of complex ideas across an immense timeframe and scope of disciplines in a succinct and highly readable format. Really looking forward to all future blog posts by this author.